CO2 Reaction Using Regenerative Energies and Catalytic Technologies (CO2RRECT)
- contact:
Dr. Thorsten Kayser
- funding:
BMBF
- Partner:
Bayer Technology Services GmbH (Projektleitung)
Bayer MaterialScience AG
RWE Power AG
Siemens AG
Invite GmbH
CAT Catalytic Center
RWTH Aachen
Max-Planck-Institut Magdeburg
Fritz-Haber-Institut Berlin
Leibniz-Institut für Katalyse e.V. an der Universität Rostock
Ruhr-Universität Bochum
Technische Universität Dortmund
Technische Universität Dresden
Universität Stuttgart
Karlsruher Institut für Technologie
Technische Universität Darmstadt - startdate:
01.10.2010
- enddate:
31.12.2013


The annual global anthropogenic CO2 emissions is in the range of 30 Gt , accounting for approximately 60% of the additional anthropogenic greenhouse effect. The focus of the CO2RRECT project is the recycling of CO2 as a raw material for the chemical industry in order to make a direct contribution to the reduction of these emissions. The CO2 as a carbon source with an unlimited availability may replace conventional raw materials of chemical industry, such as Methane. At the same time it may reduce the demand of fossil primary energy carriers.
CO2 is a thermodynamically very stable molecule whose activation requires significant amounts of energy. This is done by the reaction with H2 or CH4 with suitable catalysts at high temperatures. Within the CO2RRECT project the following processes for the conversion of CO2 to CO are investigated:
Reverse Water -Gas Shift CO2 + H2 -> CO + H2O
Dry reforming CO2 + CH4 -> 2CO + 2H2
However CO2 activation in terms of sustainability and climate protection only makes sense if the energy is based on renewable sources.
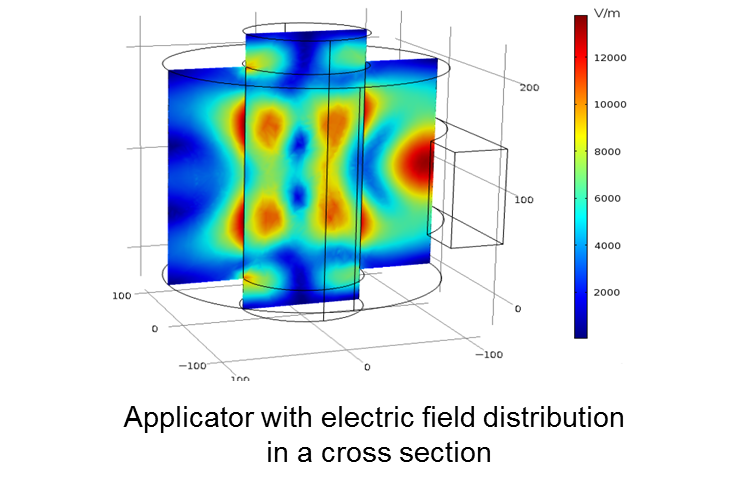
The endothermic reduction requires a continuous heat supplied. At the IHM, the use of microwave heating is examined for this purpose. Microwaves have the advantage to penetrate into the catalyst carrier, and thus provide a direct volumetric heating. Therefore the energy reaches directly the reaction site, and thus can be used more efficiently.
For the experimental investigation, a microwave applicator is built on a laboratory scale at IHM. This also forms the basis for the development of a simulation model. In this model the mutual coupling of the electromagnetic field, thermal field and the reaction kinetics must be considered. Only thus can be targeted statements regarding the energy balance and thus the efficiency of this novel method meet.
As part of the BMBF-funded project CO2RRECT, it is about the energy-efficient reduction of carbon dioxide (CO2) to carbon monoxide (CO) using a catalyst. CO is used as a precursor for many chemical products. As this reduction is an endothermic reaction, energy must be continuously supplied in the form of heat. At the IHM, the use of microwave heating for this purpose is investigated. Microwaves have the advantage that they penetrate into the catalyst bed, and thus they heat directly from inside. This way, the energy is transferred directly to the location of reaction, resulting in a high efficiency.
For experimental investigation an applicator is built at IHM in laboratory scale. This also forms the basis for the creation of a simulation model. In this model the mutual coupling of the electromagnetic field, thermal field and the reaction kinetics must be considered. Therewith only, targeted statements can be made regarding the energy balance and the efficiency of this novel method.